At the heart of drug development for cardiovascular disease
Nathaniel Huebsch plans to grow heart muscle in the lab to predict drug response with a CAREER Award from the National Science Foundation
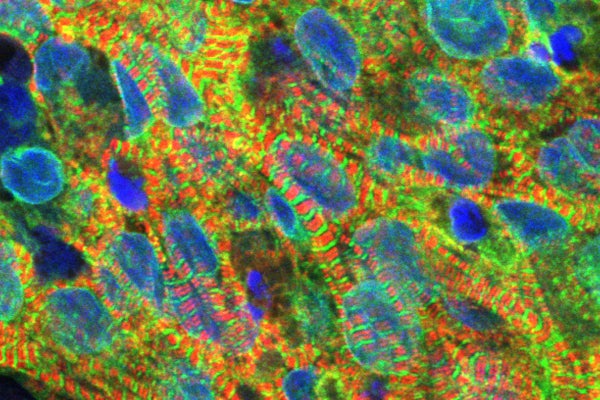
Heart disease, the leading cause of death in the United States, accounts for about 700,000 deaths annually, according to the Centers for Disease Control and Prevention. To help tackle this significant and wide-reaching health challenge, better drug treatments are needed. However, the lab-grown heart muscle used in drug development research does not accurately reflect how drugs will affect real patients, a major obstacle to developing drugs to treat cardiovascular disease.
With a five-year, $695,746 CAREER Award from the National Science Foundation, Nathaniel Huebsch, assistant professor of biomedical engineering in the McKelvey School of Engineering at Washington University in St. Louis, will grow heart muscle in the lab that is more representative of adult heart tissue and use that muscle to predict how drugs will affect patients’ hearts. CAREER awards support junior faculty who model the role of teacher-scholar through outstanding research, excellence in education and the integration of education and research within the context of the mission of their organization.
“Currently, heart muscle grown in the lab is more similar to heart muscle in a fetus than an adult,” Huebsch said. “We need to create lab-grown heart muscle that does a better job at predicting how real patients' heart muscle will respond to drugs. This is going to be especially useful for developing cures for genetically inherited diseases. It will also help us understand how to avoid cardiac side effects of drugs that are used to treat diseases like cancer, provide guidance on whether emerging therapies will work on certain diseases such as arrhythmias, and determine if there are more effective times for the application of therapies.”
Key to this endeavor is the integration of mechanical and metabolic cues during heart muscle development. Shortly after birth, babies’ hearts must pump with stronger force because their blood pressure increases. At the same time, their hearts’ energy source shifts from sugar to fat. Huebsch likens this shift to switching your car from regular to premium gas – you might not notice any difference unless you’re hauling a heavy load, or, in the case of a heart, pumping against greater resistance. By mimicking the dynamic changes in mechanical resistance that the heart muscle has to pump against, together with changes in energy source that occur during early postnatal life, Huebsch aims to enhance the maturity of lab-grown cardiac tissue.
“From our prior work, we know that the electrical behavior of heart muscle cells is exquisitely sensitive to mechanical forces, whether you’re looking at a real heart or lab-grown engineered heart tissue,” Huebsch said. “We now want to understand how the cells sense these mechanical forces and how they integrate the force sensing with the fuel source they use to produce the energy to pump against these forces.”
Huebsch’s CAREER award supports combining those factors and developing a deep, quantitative understanding of how different inputs affect heart behavior – a step he says will be critical to producing lab-grown muscle that accurately mimics real hearts.