Colored light investigated to control irregular heartbeat noninvasively
A team led by Chao Zhou in biomedical engineering will use fruit flies to study a noninvasive stimulation and imaging technique to regulate an irregular heartbeat
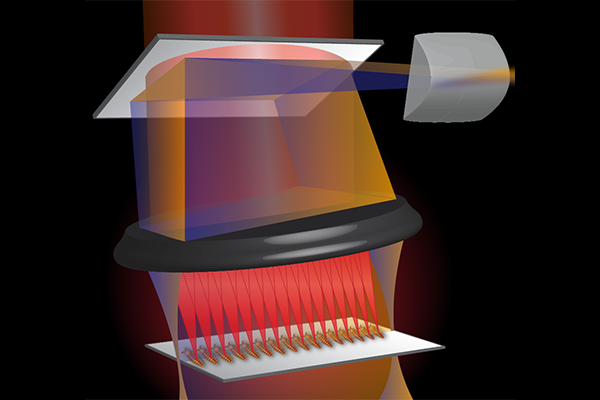
Athletes condition their bodies to withstand their sport. Researchers have taken that concept to an idea that the heart can be preconditioned to protect against a short-term loss of blood and oxygen flow, known as ischemia. Now, a team of researchers at Washington University in St. Louis will test a noninvasive stimulation and imaging method to precondition the heart for ischemia using a simple pulse of light.
With a four-year, $2.08 million grant from the National Institutes of Health, Chao Zhou, associate professor of biomedical engineering in the McKelvey School of Engineering, will lead a multi-institutional team that will apply optogenetics, a technique that uses light to control the opening and closing of ion channels, to help the heart achieve regular beating. Zhou will collaborate with Abhinav Diwan, MD, professor of medicine, of cell biology and physiology and of obstetrics and gynecology; Jeanne Nerbonne, professor of medicine and of developmental biology and director of the Center for Cardiovascular Research; and Kenneth Schechtman, professor of biostatistics and of medicine, all at Washington University School of Medicine. He also will work with Airong Li, MD, PhD, assistant professor of neurology at Harvard Medical School, and Rudolph Tanzi, the Joseph P. and Rose F. Kennedy Professor of Child Neurology and Mental Retardation at Harvard University and Massachusetts General Hospital, both experts in human and fruit fly genetics.
“The light pulses allow us to mimic different heart conditions,” Zhou said. “We can increase the heart rate, make it slower, or stop it and mimic cardiac arrest. When we remove the light, the heart goes back to the rate it had before.”
The team will use the light beams on the fruit flies at different developmental stages, from larvae to adult. The motivation behind the work is to investigate an option for pacemakers, which control the heart rate in patients with rhythm irregularities, but also affect surrounding tissues and require periodic invasive surgery to replace the batteries.
“If we can use light, we don’t have to have a direct contact with the heart and will have high specificity to express these options only in the cells we want,” Zhou said.
More recently, Zhou’s team demonstrated that a pulse of red light was able to penetrate deeper into the tissue, giving it even greater efficiency and a higher success rate for pacing the fly heart. This work was published in Nature Communications Biology in June 2020.
Zhou developed a noninvasive imaging technique known as space-division multiplexing optical coherence tomography (SDM-OCT), which enables parallel imaging from multiple sample locations simultaneously.
In this newly funded work, Zhou will develop a high-throughput instrument to integrate the noninvasive SDM-OCT imaging and optogenetic pacing techniques on the fruit fly heart. The team will use that data to create models of the physiology of the fruit fly hearts and study the changes in the heart in response to mimicking different heart conditions, including a temporary lack of oxygen to further study cardiac changes and preconditioning.
“The data from these fly studies can be used to intelligently design future studies in vertebrate models for the best potential candidates,” Zhou said. “The fruit fly is the ideal model system to take advantage of these technologies.”