Mechanism behind heartbeat regulation, heart function uncovered
New findings show how CaM and PIP2 orchestrate large-scale molecular movement of the KCNQ1 cytoplasmic domain to facilitate channel opening
In the heart, a complex set of proteins called voltage-gated ion channels coordinate proper heartbeats. Researchers at Washington University in St. Louis have uncovered new details about the function of one important cardiac ion channel, the KCNQ1 potassium channel, that regulates the heartbeat and could shed light on the causes of cardiac arrhythmia.
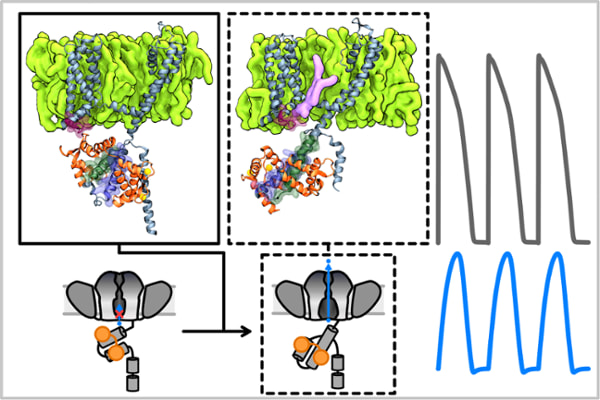
Jianmin Cui, professor of biomedical engineering in the McKelvey School of Engineering, and Po wei (Billy) Kang, an MD/PhD student in the School of Medicine who conducts research in Cui’s lab, led an international team that revealed new details on how two biomolecules, the protein calmodulin (CaM) and the lipid phosphatidylinositol 4,5-bisphosphate (PIP2), modulate opening and closing of the KCNQ1 potassium channel by combining electrophysiology techniques and molecular dynamics simulations. Their findings show how CaM and PIP2 orchestrate large-scale molecular movement of the KCNQ1 cytoplasmic domain to facilitate channel opening.
The work was published in Science Advances Dec. 11, 2020.
“We knew parts of the channel, such as the KCNQ1 cytoplasmic domain, were important in modulating channel activities, but we didn’t know how,” said Kang, the paper’s first author. “This work is a detailed analysis of calmodulin and the cytoplasmic domain of KCNQ1 to study whether large-scale conformational change is important for the ability of the channel to sense voltage and open and close in response.”
Cui said the KCNQ1 channel is very important for proper heart function and regulating heartbeat, which ranges from about 60-100 beats per minute in a normal adult. The cellular signature of a heartbeat is the action potential, with each beat corresponding to one action potential. In a stressful situation that puts people in a “fight-or-flight” state, the heart rate quickens and necessitates action potential shortening. Enhanced KCNQ1 opening triggered by the “fight-or-flight” response is a key physiological mechanism to shorten the action potential. When the KCNQ1 channel does not function well, an irregular heartbeat, or arrhythmia, can develop, which can sometimes lead to sudden death.
“This channel terminates the action potential, which terminates the heartbeat and prepares for the next beat,” Cui said. “Compared with many other potassium channels that we know of, this is the slowest channel to turn on, which is important to maintain the 60-100 beats per minute heart rate. What we found gives a clue as to why the channel turns on so slowly, partly because there is such a large interaction between calmodulin in one part of the channel, then because it has to go through a large conformational change in the cytosolic domain.”
The team, which includes collaborators in Sweden and other faculty at Washington University in St. Louis, now plans to use fluorescence measurement to detect the molecular movements of the KCNQ1 channels. Cui recently received a four-year, $160,000 grant from the United States-Israel Binational Science Foundation to continue research in this area. Previous work from his lab on the KCNQ1 potassium channel is featured in a collection of articles on ion channels in eLife.
Funding for this research was provided by the National Institutes of Health R01HL126774 (J.C.); R01NS092570 (J.R.S.); F30HL151042 (P.W.K.); the Science for Life Laboratory and the Göran Gustafsson Foundation (L.D.).
Kang PW, Westerlund A, Shi J, McFarland White K, Dou A, Cui A, Silva J, Delemotte L, Cui J. Calmodulin acts as a state-dependent switch to control a cardiac potassium channel opening. Science Advances, Dec. 11, 2020. 10.1126/sciadv.abd6798