Team looks to develop process to break down lignin
A McKelvey Engineering-led team seeks to derive fuels, chemicals and materials currently produced from petroleum to be derived from lignin, a renewable material from plants
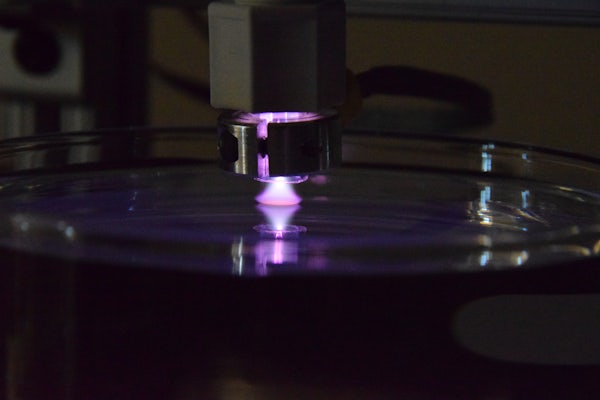
Researchers have been working to reduce dependence on nonrenewable resources, such as petroleum, and their derivative products by looking for sustainable alternatives, such as those made from plants. Lignin is an abundant material in plants that has potential for its chemical building blocks to be used in more sustainable replacements for chemicals, materials and fuels. However, lignin is difficult to break down into its smaller units in a controlled fashion for chemical production, and existing processes have not proven efficient.
A multi-disciplinary, multi-institutional team of engineers and scientists, led by Elijah Thimsen in the McKelvey School of Engineering at Washington University in St. Louis, plans to use a nontraditional chemical process to promote electrochemical conversion of lignin, a rigid polymer found in plant cell walls, into smaller, more usable molecules with a four-year, $1.7 million grant from the National Science Foundation. The team expects that a plasma-liquid interface will not undergo electrode fouling and have higher reaction rates than other conversion methods, such as using enzymes or microorganisms.
Thimsen, an assistant professor of energy, environmental & chemical engineering who works with low-temperature plasma, will be joined by co-principal investigators Marcus Foston, associate professor of energy, environmental & chemical engineering, and Kimberly Parker, assistant professor of energy, environmental & chemical engineering, both at McKelvey Engineering; Mark Mba-Wright, associate professor of mechanical engineering at Iowa State University; and Stephen Maldonado and Corey Stephenson, both professors of chemistry at the University of Michigan.
Using a catalyst developed by the collaborators at the University of Michigan, the team plans to use low-temperature plasma, a partially ionized gas, to promote carefully-controlled organic reduction-oxidation (redox) chemistry in liquid to break down lignin, which is often discarded as waste or burned for power or heat. Low-temperature plasmas provide a lot of energy to a system without adding heat. The work builds on preliminary results published in the Journal of Physics D: Applied Physics in February 2020.
“This project would provide a paradigm shift in engineering knowledge because it would conceptualize the plasma-liquid interface as a controlled and selective means by which to promote reduction-oxidation reactions of organic molecules without solid electrodes and without intentionally-added electrolyte,” Thimsen said.
The chemical process is hypothesized to be more stable than existing efforts, relatively inexpensive, both environmentally and economically, and offers potential for unique product distributions, Foston said.
“The amount of energy required to break down lignin is like hitting it with a sledgehammer —we can’t control the shape and size of the pieces that come out,” said Foston, who specializes in lignin characterization and conversion. “We are looking to develop a knife or a scalpel to cut out specific components of the lignin.”
Parker, whose research focuses on organic chemical transformation, will focus on redox reactions occurring at the plasma-liquid interface and how the investigators can control the reactions to get the desired products. In addition, Parker plans to mentor undergraduate student teams to prepare them for national design competitions.
As part of the work, the McKelvey Engineering group will host “Researcher for a Day” events with local middle school students from groups underrepresented in the STEM fields, allowing them to do an experiment with biomass and learn about the conversion of biomass to useful products.
Oldham T, Chen M, Sharkey S, Parker K, Thimsen E. Electrochemical characterization of the plasma-water interface. Journal of Physics D: Applied Physics, Volume 53, Number 16. Feb. 18, 2020.
Click on the topics below for more stories in those areas
- Research
- Energy, Environmental & Chemical Engineering
- The Institute of Materials Science & Engineering