Fine-tuning device performance with swarms of swimming cells
J. Mark Meacham’s lab uses motile algae cells to measure performance of high-tech microfluidic devices
Scientists use acoustic microfluidic devices to separate and sort components in fluids, such as red and white blood cells, platelets and tumor cells in blood, to better understand diseases or to develop new treatments. However, technologies developed in research labs often lack the consistent performance needed for use in clinical and industrial settings. A team of researchers at Washington University in St. Louis is turning the standard practice of using microfluidic tools for scientific discovery on its head by using a living organism for real-time measurement and monitoring of acoustic microfluidic device performance.
Mark Meacham, assistant professor of mechanical engineering & materials science in the McKelvey School of Engineering, used Chlamydomonas reinhardtii, a single-cell green alga that swims with two cilia, or whip-like structures, to test the efficacy of bulk acoustic wave devices created in his lab. These devices use piezoelectric materials to translate an electrical signal to mechanical vibrations, which then generate ultrasonic standing waves in the fluid-filled channel of a device. Meacham and Minji Kim, a doctoral student in his lab, design these devices to operate at multiple resonant frequencies to generate strong acoustic waves with maximum energy transfer. Efficient operation is critical because inefficient devices generate heat that can kill biological cells.
It is the first reported work to provide this functionality in real time and for a variety of device geometries. Results of the work are published in Lab on a Chip Feb. 9, 2021, and are featured on the back cover of the print journal.
“The goal of this work is to use these cells to characterize the acoustic field, to find resonances and to assess field strength, and eventually to calibrate device performance using the cells as our measurement tool,” Meacham said. “We know how much power is put in. The cells give us a way to evaluate how much of that power is useful.”
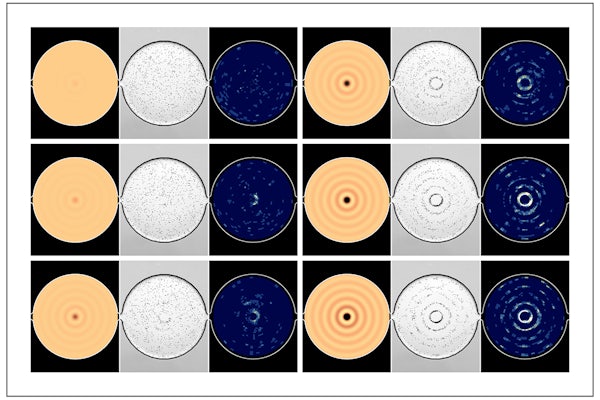
Meacham and his team first demonstrated their approach on simple device geometries, a straight microchannel and circular chamber. They then applied this knowledge to a more complex device with a microfluidic chamber made up of two different domains. The team found that the swimming cells continuously responded to the changing acoustic field, easily distinguishing between the various resonances of the chamber. These active cells are different from the passive polystyrene beads typically used in other experimental methods, a tedious process that does not provide real-time information.
Chlamydomonas reinhardtii have a fairly uniform size and swimming speed, and they naturally spread throughout the chamber of a microfluidic device to distribute themselves evenly. The algae swim at about 100 micrometers per second, moving across the device in a matter of seconds. When Meacham and Kim turn on the acoustic radiation force, the algae’s motion changes as they are trapped tightly together in low-pressure regions of the chamber. As Meacham and Kim vary the frequency of operation, the algae aggregate into different shapes, such as straight lines or bulls-eye circles, depending on the shape of the channel. When they remove the force, the cells return to swimming naturally.
“These living cells make for a very effective measurement tool to investigate acoustic forces,” Meacham said. “They are also easy to culture and don’t need a lot of specialized equipment like animal cells, just a box with a light at room temperature.”
Kim M, Bayly PV, Meacham JM. Motile cells as probes for characterizing acoustofluidic devices. Lab on a Chip, Feb. 9, 2021. DOI: 10.1039/d0lc01025a
Funding for this research was provided by the National Science Foundation and the Spencer T. and Ann W. Olin Fellowship for Women in Graduate Study.





