Sound may be key to separating molecules, cells
Mark Meacham to develop acoustic microfluidic platform to separate cells and particles
Life sciences and biomedical research relies on the ability to separate molecules and cells from biological fluids but doing so efficiently and with precise control has been a challenge. This is particularly true when the important cells, or targets, need to be isolated from a complex mixture of other microscopic particles such as in blood.
J. Mark Meacham, associate professor of mechanical engineering & materials science in the McKelvey School of Engineering at Washington University in St. Louis, plans to progress a unique microfluidic technology with a four-year, $1.5 million technology development grant from the National Institutes of Health. A prototype was first designed and fabricated as part of his microfabrication class in 2016 and was further developed by two students in his lab, Mingyang Cui and Michael M. Binkley, who earned doctorates in mechanical engineering from McKelvey Engineering in 2021 and 2019, respectively.
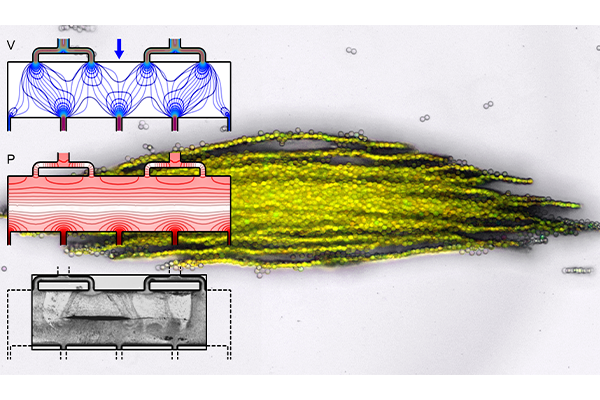
The technology is based on a microfluidic subunit that controls the motion of very small objects, from the microscale to the nanoscale, for separation, enrichment or confinement. The subunit supports an acoustic wave with low pressure “traps” oriented perpendicular to the direction that a fluid is flowing, allowing size- and material-based selection of target cells or particles. Subunits can be repeated in different configurations to meet yield or volumetric throughput requirements while also allowing for continuous visual monitoring of the trapped cells or particles.
“These features of the technology differentiate it from conventional acoustic separations devices because it decouples the sample throughput from the acoustic trapping or separation capability,” Meacham said. “This can be a major improvement that opens lots of different applications due to the scalability and versatility of the core subunit technology. This grant will allow us to explore various applications that use different device formats.”
When used for imaging, ultrasonic waves bump off of materials with different physical properties to create a picture of the structures inside the body. In acoustic microfluidic devices, this “bump” can be used to apply forces to cells or particles to move or trap them. Separating specific cells allows researchers to isolate, for example, circulating tumor cells or pathogenic bacteria from blood. When separated particles are then trapped in a predefined location, they can be exposed to different environmental conditions like chemicals or changing temperature and monitored. Meacham’s technology has demonstrated this functionality in his lab’s initial work.
With the technology development grant, Meacham and his team will optimize key components such as the microfluidic channel geometry, which consists of perforated walls made up of arrays of pillars. They will investigate how different wall geometries, microchannel layouts and operating conditions impact trapping efficiency. The perforated walls are important to generate sufficient acoustic forces to trap particles while providing a wide fluid flow path to avoid clogging, which can be an issue for other microfluidic separations devices.
“Our experiments have proven that you can use a perforated wall to allow flow through the pillars while being solid enough to reflect waves to set up a standing acoustic wave field,” Meacham said. “To release particles for additional downstream analysis, you simply turn off the field.”
Meacham’s project has been previously funded by a 2017 Collaboration Initiation Grant from the Engineering School in collaboration with Mikhail Berezin, associate professor of radiology and director of the Optical Spectroscopy Core Facility for Mallinckrodt Institute of Radiology at Washington University School of Medicine. The new project also supports an ongoing collaboration with Arpita Bose, associate professor of biology in Arts & Sciences, building on work funded by the U.S. Department of Defense. The technology has received two patents with assistance from the university’s Office of Technology Management and has been the subject of three journal articles.




