Cells take on dual identities with competing factors trapped in the nucleus
Research in Amit Pathak’s lab reveals new cell migration behavior
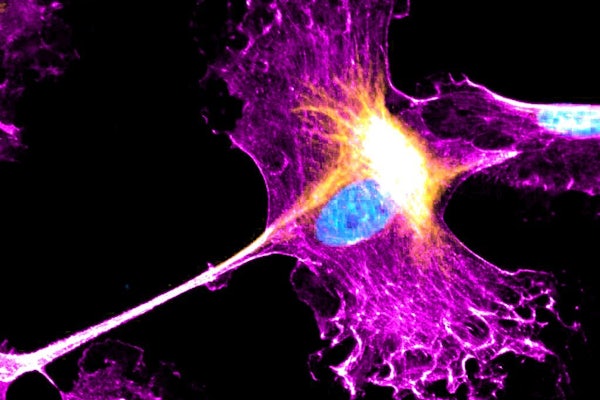
Cells migrate to different tissues for a variety of reasons, including organ development, tissue repair and the spread of cancer. Researchers in the McKelvey School of Engineering at Washington University in St. Louis have found unexpected activity in the nucleus of healthy cells that provides new insight into cell mechanics.
Amit Pathak, associate professor of mechanical engineering & materials science, working with Carly Krull, a doctoral student in biomedical engineering, and Haiyi Li, who earned a bachelor’s degree in computer science & engineering in 2022, found that when they gave the cancer drug Leptomycin B to healthy cells, the cells stopped growing, but several competing genes in their nuclei became active.
“All of a sudden, everything is happening in the nucleus,” Pathak said. “The factors that slow down the cells, the factors that make the cells faster, the factors that make the cells cohesive and the factors that generate forces in cells all became active. All of these factors are normally competing with each other, and they all became active together.”
Results of the research were published in eLife Feb. 21, 2023.
Cells can change properties from epithelial, which is one of the main types of tissue in organs, to mesenchymal, both dynamically and stably according to requirements of the body. While the two types of cells are very different, epithelial cells tend to stick together in groups and can go through an epithelial-mesenchymal transition that helps them develop into other tissues and organs. The transition can also be reversed.
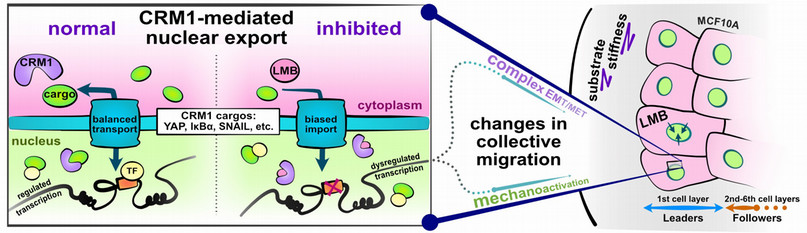
Leptomycin B is in a class of drugs known as nuclear export inhibitors designed to block export of a variety of proteins from the nucleus into the cytoplasm of the cell. In months of closely-watched experiments giving Leptomycin B to healthy human mammary epithelial cells, Krull and Li assessed their migration characteristics and changes in their epithelial-mesenchymal features. They observed that the cell populations became both more epithelial and more mesenchymal, and cells migrated faster across tissue-like soft surfaces.
Before this work, it was inconceivable that cells could be both epithelial and mesenchymal concurrently, Pathak said.
“In cancer, the goal is to make the cells cohesive and not migratory or mesenchymal, and if they migrate, you kill them,” Pathak said. “We saw that these cells became cohesive, forming streams, and even though there was chaos in the nucleus, the cells stuck together and expedited their migration.”
Krull said their findings showed that inhibiting nuclear export can strengthen both epithelial and mesenchymal characteristics.
“These findings could shed light on how cells with disease-like transformations might perform seemingly contradictory tasks, like migrating fast while holding together,” she said. “Since nuclear export inhibition is being optimized to treat cancer and has been shown to reduce cancer cell invasion, we expected migration in our system to reduce exclusively. We were surprised to find that such strategies actually elevated cell migration on soft substrates.”
“This was quite a puzzle to solve, and no one has shown this before,” Pathak said. “If the focus was always to shrink the tumor, we would have never found these other phenotypes.”
Krull said the team is now working to understand the time scales of nuclear export-driven cell-state transitions and how these transitions can be molecularly controlled.
Krull CM, Li H, Pathak A. Nuclear export inhibition jumbles epithelial-mesenchymal states and gives rise to migratory disorder in healthy epithelia. eLife, Feb. 21, 2023. DOI: https://doi.org/10.7554/eLife.81048
This work was supported by the National Institutes of Health’s National Institute of General Medical Sciences (R35GM128764).




