How a handful of stray atoms led to a new material that curves light in a record-setting way
A collaborative team has created a unique, record-setting material that can bend one infrared ray of light in two directions
A team led by scientists and engineers from the University of Wisconsin-Madison, the University of Southern California and Washington University in St. Louis, has created a unique, record-setting material that can bend one infrared ray of light in two directions.
For the second time in five years, the team created a crystal with the highest degree of what’s known as “double refraction” on earth. This time they beat their own record, and the new higher-performing crystal could lead to innovations in night vision, Lidar, chemical sensing, microscopy, and many other applications.
The work was published September 20, 2023 by the journal Advanced Materials. The UW-Madison leads are Hongyan Mei, an electrical and computer engineering PhD student, and Jad Salman, a 2022 PhD graduate, both from the group of Mikhail Kats, associate professor of electrical and computer engineering.
When light travels from one substance to another—for instance, from air into water, or water into glass—it slows down by a predictable amount, and that causes the light to bend. This bending is called refraction. Double refraction happens when that ray of light enters an anisotropic material—one with properties that are different depending on direction—and splits into two rays, each headed different ways. Imagine a bundle of rods grouped together, with one refractive index, or measure of its bending ability, down its length, and another in the perpendicular direction. The difference between those two refractive indices is called birefringence.
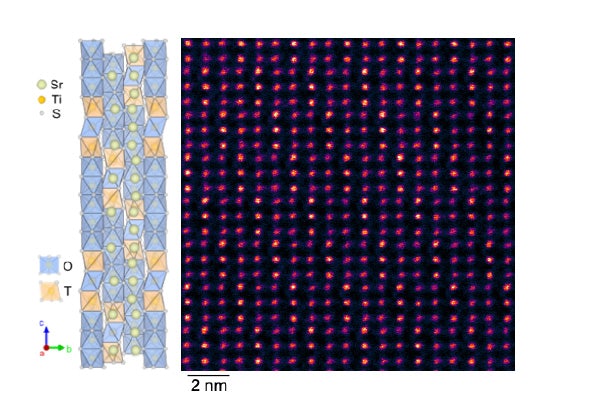
Despite the team’s previous experience with record-setting birefringence, Kats and his group were surprised to discover that the birefringence in the new material, called strontium titanium sulfide (Sr9/8TiS3 or STS), is 3 times the birefringence of their previous record-holder, barium titanium sulfide (BaTiS3 or BTS), which should have had a similar structure.
Why, exactly, was a mystery. After an atomic-level examination of the strontium titanium sulfide, grown by PhD student and co-lead author Boyang Zhao and colleagues at USC, the team found that it had a few more strontium atoms than expected. Those extra handful of atoms made the difference: They caused the crystal to have a much larger repeating structure, or periodicity rather than a single, unchanging crystalline structure.
Colleagues at Washington University in St. Louis, including PhD student and co-lead author Guodong Ren, examined this arrangement, coming up with atomic-scale theories for the huge optical anisotropy.
“It turns out that when you have this particular crystal arrangement, the structure results in an enhanced refractive index along one direction of the material,” says Kats. “This was very, very unexpected. You generally don’t expect such a small atomic-scale change to result in such a large difference in optical properties.”
Read the full story here.





