Water: Vital for life
Washington University's efforts are playing a major role in solving the world's water crisis

One of the world's most precious resources is essential for public health, food supply and the economy."Water is the most basic of all resources. Civilizations grew or withered depending on its availability."— Dr. Nathan W. Snyder
Most of us take for granted that when we turn on a faucet in our homes or businesses that clean, fresh, drinkable water will be available in a seemingly endless supply. But in the last several years, clean water has become almost a luxury in parts of the U.S. due to drought and changes in climate, while worldwide, there is too much water in some places and not enough in others.
One of every six people in the world does not have access to clean water, making this shortage responsible for more deaths worldwide than war, according to the National Academy of Engineering (NAE). Lack of clean water has a significant effect on public health, food supply, manufacturing and the economy. That's why providing access to clean water is one of the NAE's 14 Grand Challenges for engineering in the 21st century.
In the past few years, several areas of the U.S. have experienced threats to their water supply. Most recently, after residents of Flint, Mich., complained of the odor and color of their drinking water, officials determined that the water was contaminated with lead that exceeded the U.S. Environmental Protection Agency's limit of 15 parts per billion. In West Virginia in 2014, a chemical used to clean coal leaked from a storage facility into the Elk River, which served as the water supply for more than 300,000 residents.
In addition to water quality, water supply is in peril. In the U.S., more than 33 million people in Arizona, California, Colorado, New Mexico, Nevada, Utah and Wyoming share portions of the Colorado River for their water supply, of which the majority goes to agricultural use. However, population growth in these states and changes in climate have led to demand for water exceeding supply, leading to severe drought conditions in California since 2011. As one of many steps to alleviate the crisis, Californians passed a $7.5 billion water bond measure in 2014 that included $2.7 billion for increased water storage.
In June 2013, President Barack Obama rolled out his Climate Action Plan that proposed conserving water resources, managing drought and drought-related risks and preparing for future floods. In addition, the plan calls for strengthening government and community planning and response, such as improving water use efficiency and increasing water storage to manage changes in water supply, as well as using drought-resistant seeds to help farmers manage changes in climate.

Engineering’s role
Engineers in the School of Engineering & Applied Science are working to meet some of the challenges in the President's plan as well as the NAE's Grand Challenge. By addressing the issues from a variety of angles, Washington University's efforts are playing a major role in solving the world's water crisis.
In the Department of Energy, Environmental & Chemical Engineering, the Engineered Aquatic Processes group includes John Fortner, PhD, the I-CARES Career Development Assistant Professor; Daniel Giammar, PhD, the Walter E. Browne Professor of Environmental Engineering; and Young-Shin Jun, PhD, the Harold D. Jolley Career Development Associate Professor. The trio creates a world-class group of researchers tackling water issues from unique angles.
Jun studies natural and engineered aquatic processes, including the impact of human behavior on water quality and supply. Currently, she and her research team are studying managed aquifer recharge (MAR), one important technical solution available to address water needs in areas where supplies are low.
"We are using more and more water, and, somehow, we have to refill the aquifers," Jun says. "By injecting reclaimed water from water treatment systems into depleted aquifers, we can keep them filled."
Jun's group also examines whether there are any adverse effects on the water quality from replenishing groundwater aquifers. Results from her work will help determine how engineers should pretreat reclaimed water from wastewater treatment plants to minimize these adverse effects, providing a basis for developing more sustainable MAR operations. In addition, knowledge obtained from the study can be applied to other environmental systems, including areas with arsenic-contaminated surface or groundwater. Her group's recent publication on MAR was recognized as the 2014 Top Environmental Science Paper from Environmental Science & Technology.

Orange County, Calif., has been using MAR to replace some of the water that is pumped from nearly 400 wells in the area. In the last decade, the county's water district had the foresight to complete a groundwater replenishment system that purifies wastewater for recharge year-round. This has become particularly important because water from its traditional sources, including the Colorado River, has become scarcer in the last few years.
Jun's group also investigates reverse osmosis membrane modifications to use during the desalination of seawater or brackish water to generate fresh water.
Giammar focuses his research on chemical reactions affecting the fate and transport of heavy metals, radionuclides and other inorganic contaminants in natural and engineered aquatic systems. He is particularly interested in reactions at solid-water interfaces that are relevant to drinking water treatment.
Giammar is teaming with Jeffrey Catalano, PhD, associate professor in the Department of Earth & Planetary Sciences, to study the performance and mechanisms of iron electrocoagulation on two projects: one funded by the National Science Foundation (NSF) to use the technique to remove hexavalent chromium from drinking water, and one funded by the university's Consortium for Clean Coal Utilization (CCCU) to remove selenium from wastewaters from coal use. Previously, he used the same technique to remove arsenic from drinking water in collaborative work with the Indian Institute of Technology Bombay.
"They had taken field measurements, but hadn't taken them into the lab, so we brought their data into our lab," Giammar says.
"It was a merger of fundamental chemical investigation with a very applied problem in a setting where water quality is incredibly important." — Daniel Giammar, PhD

Fortner's research focuses on the environmental implications and applications of advanced materials. With Pratim Biswas, PhD, the Lucy & Stanley Lopata Professor and chair of Energy, Environmental & Chemical Engineering, he has developed a one-of-a-kind multifunctional membrane, or microfilter, with crumpled graphene oxide-based nanocomposites, which are highly water-permeable, photoreactive and antimicrobial.
"These nanocomposites are similar to a basket full of crumpled paper — they are inherently porous," Fortner says. "At the nanoscale, water can move through it better than most commercial membranes used now."
In addition, Fortner's lab makes other nanoparticles with different compositions and surface coatings. One nanoparticle Fortner developed binds uranium in water at the highest level ever reported for an engineered sorbent.
In 2014, Fortner and Giammar began a collaboration to use a novel technology using nanoparticles to remove metal and metalloid contaminants, including arsenic, uranium and chromium, from drinking water. They are engineering nanoparticles that will adhere to the grains of sand in a traditional sand-based water filter. As contaminated water flows through the filter, the engineered particles can adsorb dissolved pollutants, thus treating the water. Once the nanosorbents are used to capacity, they can be selectively removed from the sand grains and collected with a magnet for waste disposal. Fresh nanoparticles can then be added to the sand to regenerate the filter.
Fortner and Giammar also teamed with Maxine Lipeles from the School of Law to study the energy-water nexus with funding from the university's McDonnell Academy Global Energy and Environment Partnership (MAGEEP). This interdisciplinary project focused the emerging energy-water nexus related to enhanced natural gas extraction and geothermal (energy-harvesting) use. Both processes are expected to play increasingly important roles in the energy resources of the U.S., India and Turkey.
WashU also is tackling water and related issues through funding from the International Center for Advanced Renewable Energy and Sustainability (I-CARES), which awards grants for interdisciplinary research on energy, environment and sustainability.

Jun has I-CARES support to work with Srikanth Singamaneni, PhD, associate professor of mechanical engineering & materials science, to develop novel, multifunctional membranes for safer, efficient and renewable-energy-driven water treatment using gold nanostars. Taking advantage of the photothermal capability of these nanostructures, Jun and Singamaneni are working together to create a new membrane that would reduce mineral scale formation and organic accumulation, while also preventing microbial buildup.
"Using these photothermal gold nanostars and light, we can kill microorganisms on a membrane," Jun says. "The membrane will use low-energy near infrared LEDs to activate the bactericidal capability. When the light shines on the gold nanostars, they convert light into heat energy by electron oscillation and relaxation. The temperature will be high enough to kill microorganisms and greatly reduce biofilm accumulation. We can also modify the membrane to repel the inorganic and organic compounds simultaneously. Currently, we are searching for more economically sustainable and earth-abundant materials to power this development."
Fortner is working on an I-CARES-funded project with Parag Banerjee, PhD, assistant professor of mechanical engineering & materials science, to create next-generation, magnetic, photocatalytic nanocomposites for use in water treatment plants. Giammar was a co-investigator on an I-CARES funded project with Zorimar Riviera-Núñez, PhD, and Lora Iannotti, PhD, both assistant professors in the Brown School, to evaluate the environmental contaminants that affect water quality in Haiti. Giammar tested water samples from sources of water in several neighborhoods in Cap-Haïtien to determine the contaminants in the water.
The future
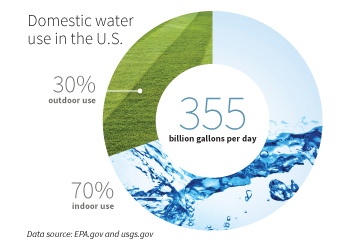 While engineers and scientists do their part to ensure the world has access to clean water, Jun says everyone is responsible.
While engineers and scientists do their part to ensure the world has access to clean water, Jun says everyone is responsible.
"We'll never be able to solve the problem unless we adjust our desire to use a lot of water, but our lives are fundamentally reliant on water," she says.
"It's everyone's responsibility to think about what we can do better." — Young-Shin Jun, PhD
Fortner says solving the water issues isn't a question of technology, but of political will and financing.
"The west of America is very dry, but parts of the Northwest are actually rainforest, so it's about how to manage and allocate and buffer, because climate change will change rainfall and snow patterns, and thus change our 'water' reality."
"What do you think is the biggest issue facing water today?"
I think it is providing the necessary amounts of high quality water under increasingly water stressed conditions. By water stress, I mean the lack of available quantities of water or such degradation of water sources that it’s not usable. We’re blessed where we are in the country, but there are other places, like California and Texas, that are starting to have to look early on at direct potable reuse. It can be done, but it’s going to take technology.
— Dan Giammar, PhD
That we take it for granted. In some places, that’s fine, but in some places, it makes the socioeconomic or geopolitical push for research or conservation or management of water more difficult. It’s not a question of technology, but the political will and money to do that and what that means for a country or region that’s always suffered, and changing that. People are afraid of change for different reasons, whether it gives people more power or a voice to women who can have more time on their hands to take care of sick children, or gives children a chance to be educated.
— John Fortner, PhD
Fresh water supply is biggest issue. It’s an old problem, but we’ll never be able to solve the problem unless we suppress our desire to use a lot of water, but our life is heavily reliant on water. Science and engineering can do desalination to turn saline water into fresh water, and we can secure ground water by recycling wastewater to return it to ground, but that has limitations unless people change their lifestyle. But this isn’t just for scientists and engineers, this is everyone’s responsibility to think about what can we do better — to make sure that we have open ground, not covered by concrete and make decisions based on building materials, gardens, and environmentally friendly materials.
— Young-Shin Jun, PhD
