Is the future of energy stuck in the past?

In the days after Hurricane Maria ravaged Puerto Rico, most of the U.S. territory had no running water or electricity. Damaged roads, harbors and airports hampered relief efforts. What's more, without power, the vast majority of cell towers and land lines were inoperable, meaning communications were all but cut off. Gas stations were running out of fuel, forcing people to ride bicycles or walk for miles in search of a fill-up.
The unfolding humanitarian disaster was a reminder not only of nature's power, but of modern society's dependence on electricity and fuel, for everything from refrigeration to transportation.
"Fossil fuels offer something very unique: energy where you want it, when you want it, in all times in all places. That's unprecedented," said Richard Axelbaum, the Stifel & Quinette Jens Professor of Environmental Engineering Science. "How do you replace something that is available to you at all times in all places, with things that aren't available to you at all times and in all places? That's the challenge facing us."

Modern society developed in large part because of relatively easy, cheap access to fossil fuels, Axelbaum argues. And the world is still using those resources, based on technologies first developed more than a century ago. From efforts to improve automobile fuel economy to reducing emissions at electrical power plants, the future of energy is inherently still in the past, according to School of Engineering & Applied Science experts. Replacing atmosphere-warming coal and crude oil, or eliminating their emissions — and doing so in a way that minimizes disruption to daily life and commerce — is not going to be easy. But the university is on the forefront of efforts to do so.
Researchers are growing bacteria to produce biofuels that can use existing pipelines and infrastructure; designing new batteries and storage tanks that can harness the power of the wind and the sun; and building technology that not only diverts carbon dioxide from the atmosphere, but allows it to be used anew. Dramatically reducing carbon dioxide emissions to levels agreed upon in the Paris climate accord will not happen without dramatically changing the way we imagine and use energy, experts say.
"You can't get there by tweaking today's technologies. It won't happen," said Jeff Phillips, who earned a bachelor's degree in mechanical engineering in 1981 and now is a senior program manager at the Electric Power Research Institute who formerly worked for Royal Dutch Shell.
“There needs to be a fundamental shift in the way we use fossil fuels and reducing the cost of low-carbon energy sources like wind and solar and nuclear. WashU is actively involved in many areas helping to bring about this fundamental shift and move toward revolutionary approaches in how to harness and use energy.”
— Jeff Phillips
Coal

Even in 2017, the vast majority of the energy that powers computers, moves cars and drives society is derived from some form of combustion. In a power plant, coal or natural gas (or nuclear decay) is used to warm water, generating steam that spins a turbine to produce electricity. In a car, an internal combustion engine burns gasoline with air, which expands to push a piston that turns a crankshaft, which turns the wheels.
Most power plants are designed and built to last at least three to five decades, and power plant operators expect to recoup their construction costs over many years. In the United States, natural gas production has spiked while coal-burning has declined, but developing economies in China, India and elsewhere are still reliant on coal. That's one reason why Axelbaum focuses on making it cleaner to use. He is working on pressurized oxy-combustion, a method of recovering the latent heat in a power plant's steamy smokestack and using it to increase the efficiency of the plant while virtually eliminating all emissions. The pressurized carbon dioxide (CO2) can later be injected deep underground, preventing it from escaping into the atmosphere and worsening global warming. Axelbaum has received more than $8 million from the Department of Energy (DOE) to develop the technology, which is being tested in a large, three-story experimental facility on campus and studied on the International Space Station, where microgravity allows for detailed studies of combustion. He says his method cuts the cost of carbon capture in half.
“It’s difficult to expect developing countries to pay double for their electricity, or use unreliable sources of energy, so we need to think about how to bring the price of clean energy down. This new technology allows us to do that.”
— Richard Axelbaum.
Prices are a concern in the U.S. as well as developing countries. But the cost of increasing access to energy may be one developing economies are willing to bear. Britain and France have announced intended rules that will bar automakers from selling petroleum-based cars after 2040, and China is considering similar rules, according to Vijay Ramani, the Roma B. & Raymond H. Wittcoff Distinguished University Professor of Environment & Energy. And in India, the government is stepping in to electrify the nation.
enerdata.net
On Sept. 25, Indian Prime Minister Narendra Modi launched a 163.2 billion-rupee (US$2.5 billion) program to ensure electricity for every household by March 2019. It will focus on helping people, including residents of more than 3,000 un-electrified villages, obtain "last-mile" electricity connections at no cost.
Still, replacing giant pieces of the power grid is often more expensive than building them right in the first place. In that case, Axelbaum's pressurized oxy-combustion and other forms of carbon capture, storage and utilization can help.
But the next generation will face a different sort of challenge, down to their very bones, Phillips says. Power plants are not designed to switch on and off, and when they are forced to do so, they can break down more easily. The metals that make up a power plant, from ventilation and tubing to the turbine blades themselves, expand and contract as they heat and cool. This causes damage due to a phenomenon known as thermal fatigue.
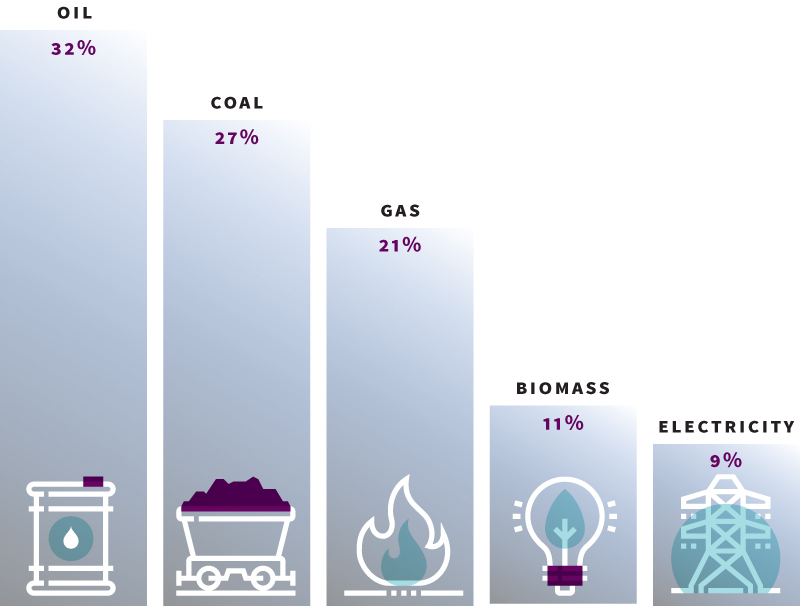
The coal share of total world energy consumption is expected to decline significantly, from 27% in 2015 to 22% in 2040.
"These old plants we have today were designed assuming they were going to run around the clock," Phillips said. "But if we say, 'Actually, new power plants that are going to use fossil fuels are going to go up and down like a yo-yo,' we need to be able to handle that. It really comes down to developing materials that have a better ability to withstand temperature swings."
Renewables

In the near future, those on-off temperature swings may become more common, as coal-fired power plants will only be needed according to the whims of nature. Pratim Biswas, assistant vice chancellor of international programs, chair of the Department of Energy, Environmental & Chemical Engineering and the Lucy & Stanley Lopata Professor, is working on technology that bridges the old and the new.
In the past few years, a new class of solar energy devices called perovskite cells, which are hybrids of organic and inorganic material, has revolutionized solar energy research. Previous generations of solar panels were made from silicon, which remains expensive, but perovskite cells could make solar panels a feasible option for anyone, Biswas said. They would be lower-cost and higher-efficiency, but right now, they don't last as long as traditional photovoltaic cells.
"A key requirement in this case is that systems be more stable, so they last for 15 to 20 years on the roof of a building," Biswas said. "They are not there as of yet. We are exploring techniques to make perovskite solar cells that are more stable."

Meanwhile, there are other ways to harness the sun's energy for power generation. Biswas is building novel materials that can capture carbon dioxide emitted from power plants and use energy from the sun to turn it into a product that can be used again. It may sound revolutionary, but it's what nature already does, Biswas pointed out.
"It's carbon recycling, but in an engineered, faster manner," he said. "Carbon is recycled by plants and trees, but it is slow."
In October 2017, St. Louis joined 46 other cities across the U.S. committing to 100% clean energy by 2035.
Renewables are the world’s fastest-growing energy source, with consumption increasing by an average 2.3% a year between 2015 and 2040.
As an aerosol scientist, Biswas focuses on tiny particles that remain airborne and can contribute to global warming. His lab's aerosol reactors engineer tiny particles and pair them with chemical catalysts, which can speed up reactions between CO2 and sunlight. The engineered particles are deposited as thin films through which air flows. When activated by sunlight, the particles trap the carbon dioxide molecules and can convert them into compounds such as simple hydrocarbons or alcohols that can be used later. Biswas' lab is filled with artificial-sunlight lamps shining on thin films filled with aerosols.
Biswas said the technology already works, but large-scale progress has been limited by policy. The U.S. does not control carbon dioxide emissions, and regulations put in place by the Obama administration face legal challenges. With tighter carbon emission rules, power utilities would have an incentive to invest in the technology, but for now it remains expensive, Biswas said.
"If regulations stepped up, this could be utilized," he said. "In countries such as India and China, where there are regulations, this is where our partnerships through the McDonnell Academy Global Energy and Environment Partnership (MAGEEP) come in handy. There needs to be a global willingness to address the CO2 issue in the most significant manner possible."
Biofuels

U.S. regulations have called for improvements in fuel economy, specifically for cars, which are still a leading cause of carbon dioxide emissions around the world. But so far, no one has come up with a replacement for gasoline that is cheap, easy to transport, fast to fill up and efficient to burn, said Fuzhong Zhang, associate professor of energy, environmental & chemical engineering and director of the Biomolecular Engineering and Synthetic Biology Laboratory.
"We need replacements with properties similar to fuels that we pump into buses, airplanes, cars and trucks," he said. "We also need the fuel replacements to be produced from cheap and renewable feedstock. But how to convert cheap and renewable feedstock to fuels like we already use is a challenge."
Although ethanol has been produced by fermenting corn and used as an additive in combustion engines, it's corrosive to both existing engines and transportation pipelines. Butanol, another form of alcohol that is less corrosive and was believed to be a better replacement than ethanol, can only replace gasoline in limited quantities and provides less combustion heat than gasoline.
"We want to go to a gas station, and in one minute, put enough energy in our cars to drive 300 miles," Axelbaum said. "It's taken for granted, but that's an unbelievable capability that we have. Fossil fuels have gotten us used to a lifestyle that's very hard to give up, but they are also very hard to replace."
Zhang is working on another organic solution: Engineering bacteria to convert sustainable resources to chemicals such as petroleum-derived gasoline, diesel and jet fuels.
"We collaborate with other WashU labs to develop processes that can convert waste biomass from agriculture into simple chemicals or sugars, and then the engineered bacteria will be able to eat them," Zhang said. "The bacteria will at the same time produce better fuels that can be directly used in current engines without further modification. We also develop methods to control these bacteria so that they would grow and do their job efficiently and robustly, in large fermenters with thousands of tons of scale."
In addition to studying the waste-conversion process, Zhang is modifying the genomes of several types of bacteria to produce new forms of materials and various chemicals with novel properties. Ideally, their advanced fuels would be pumped through the existing oil and gasoline infrastructure. One day, motorists could pull up to a pump and inject their cars not with gasoline derived from the Paleozoic, but from decidedly modern microbes.
Storage
The batteries in bacteria-burning cars will also likely look different in a few years, Ramani said. Basic car batteries have not changed much since cars were invented; indeed, the fundamentals of battery technology have not changed much since the 18th century, he said. Advances in lithium-ion batteries, which pack a high-density punch in a small size and are rechargeable, have enabled the explosion of personal tech. But for cars and, more importantly, the power grid, batteries have a long way to go.
Today, more than 35 percent of energy use in the U.S. comes from petroleum-based sources, Ramani said, quoting estimates from the U.S. Department of Energy. Of that, about 72 percent is in transportation.
"We need to electrify the transportation sector. It has to be done," he said.
The biggest advantage would be eliminating distributed sources of emissions, he adds.
"It's really hard to regulate CO2 out of a tailpipe. But then you're shifting the emission to a power plant, where the electricity is being generated. And then you can envision a way to capture it," Ramani said.
Automakers are designing improved batteries that would make electric cars more affordable, increasing their presence on American roads. Electric cars represent about 1 percent of the U.S. auto market, Ramani says. He is working with Nissan to design more efficient and durable fuel cells, which could be used in fuel cell electric vehicles or as a range extender in electronic cars such as the Nissan Leaf.
Even if all cars were electric, the energy sector would need new battery technologies. Renewable energy, especially wind and solar, is intermittent, so power plants would need some kind of backup for times when the wind is not blowing or the sun is obscured.
"If you want a really high level of penetration, you need a buffer, and that's storage," Ramani said.
He and his colleagues are working on what's known as a redox flow battery. At its heart, a flow battery's energy comes from dissolved metals that are stored outside the battery, rather than solid chemicals like lithium-metal oxides or lead-oxide that are stored inside. They require huge tanks of liquid — but at a solar or wind farm, space is generally not a problem. Each tank would contain a battery and two tanks — each full of a particular type of dissolved metal salt. In one example, a flow battery contains iron and chromium salts, which are dissolved in acid. When pumped through the battery, the iron will accept (or lose) an electron. While the chromium will lose (or accept) an electron, the amount of charge stored scales with the concentration of the salt and the size of the truck. The flow of charge can be reversed again and again, Ramani said.
"If you use elements like iron and chromium, they never degrade," Ramani said. "They will last as long as you want it to. The design lifetime for these batteries is about 20-25 years. There is really no downside to it, but we are still not there on cost."
Ultimately, money may be the main barrier — and the main driver — for moving the future of energy out of the past, Ramani said. Future energy breakthroughs will be driven in part by technology, and in part by policies that force change. Unlike fossil fuels, they may not be cheap — but someday, they might be just as abundant.
“There’s a saying in the energy industry: No power is more expensive than no power,” Phillips said. “The folks in areas that have been hit by a hurricane definitely agree with that. They would probably pay virtually any price for a chance to charge up their cellphones. So I would say reliability is even more important than cost.”
Back to Engineering Momentum